In the early days of the pandemic, authorities quickly realized that testing was indispensable to monitor the spread of COVID-19 and help inform public health policy.
Most laboratories in the US have relied on the gold-standard, polymerase-chain-reaction, or PCR test, to identify confirmed cases of COVID-19 infection. But as cases surged nationwide in the spring, PCR supply shortages resulted in significant delays in test turnaround times and a lot of people unable to get tested.
To help reduce transmission of the virus, PCR test results need to come back within two days. In the spring and summer months, labs across the country were reporting turnaround times that were days, sometimes weeks long. Delayed test results meant that people who were waiting for their results spent days—while they were potentially infectious—not knowing whether they had COVID-19. These lags also made it very hard for people to correctly follow quarantine and isolation protocols.
So in May, many states started using antigen testing, commonly known as rapid testing, to detect current infections. Around the same time, serology tests, which identify antibodies produced by the immune system from previous infections, were growing in popularity. At the same time, local and state officials continued to call for larger numbers of more accurate tests with quick turnaround times. As businesses and schools were shutting down, a testing strategy called pooled testing—intended to expedite the lengthy PCR testing process by analyzing multiple samples at the same time—seemed to occupy many minds and headlines.
Test types can be categorized in two ways: viral tests, which detect current infections, and serology tests, which identify past infections. Each testing technique has pros and cons, and their effectiveness depends on the stage of someone’s infection and the severity of the pandemic in the state they are in. Here, we explain how each test type works and compare their advantages and disadvantages. (Our “availability” metric below refers to geographic availability—how many US states and territories offer the test—rather than to how easy it is to get a given test. Access to testing varies tremendously across jurisdictions and fluctuates based on changes in supply and demand.)
Viral Tests (Diagnostic)
PCR — Polymerase Chain Reaction
THE GOLD STANDARD
PCR testing has existed since the 1980s and, until mid-spring, was the only testing method to diagnose cases of COVID-19 in the US. Also known as a molecular test, this approach detects the virus’s genetic materials and can identify cases even before symptoms emerge.
A PCR test searches for pieces of genetic material that are unique to SARS-CoV2, the virus that causes COVID-19. Once that unique region is found, enzymes in the PCR mixture then make copies of the virus’s genetic material until there is enough signal to be detected on a machine, which checks if the signal crosses the “positive” threshold.
Considered the “gold standard” in the US and around the world, PCR is the most accurate diagnostic test option, although testing errors can occur. Accuracy comes at a high price since this method requires specialized equipment and materials. Turnaround times can also be lengthy, minimizing the utility of the test as a tool to guide individual and government decision-making.
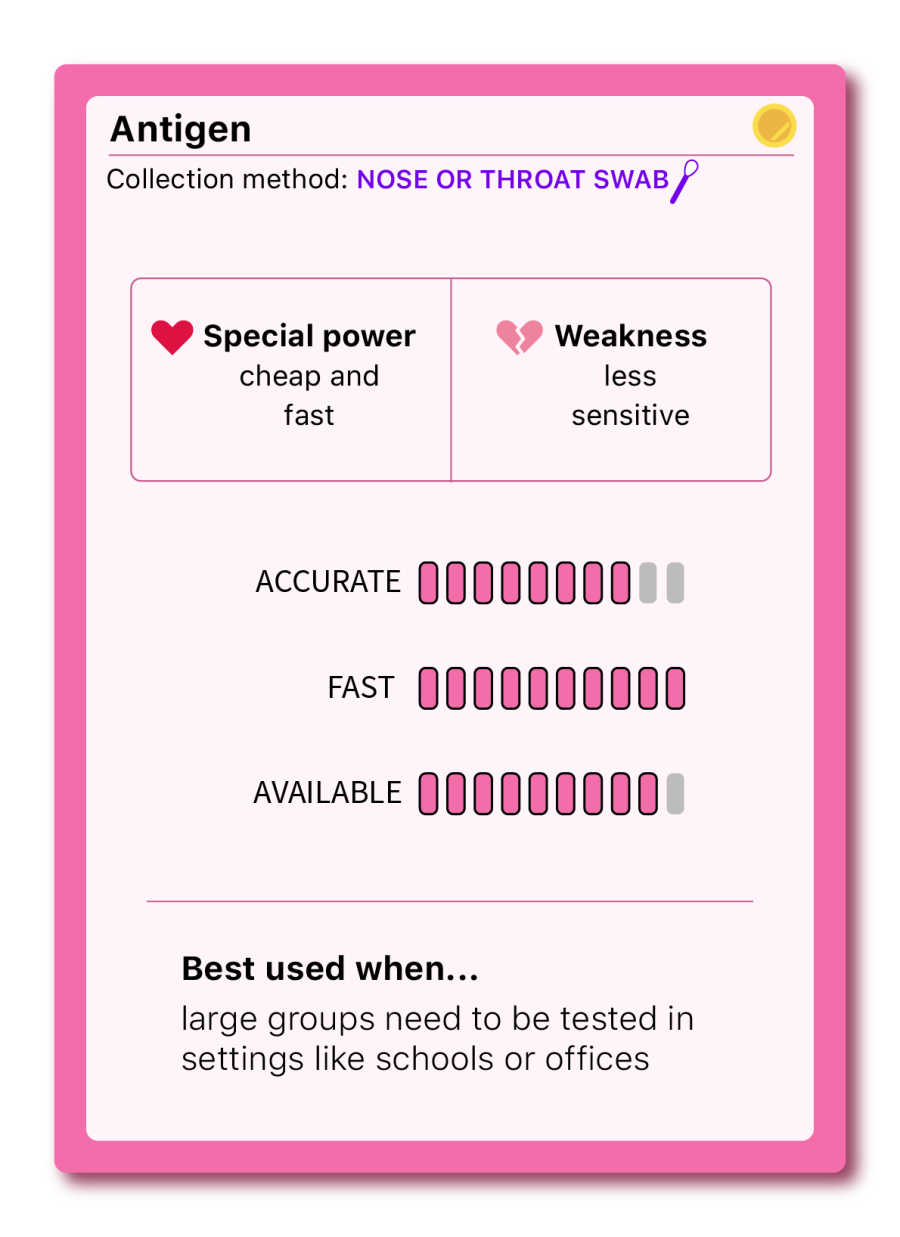
Antigen (rapid test)
THE QUICK AND EASY
At the end of the summer, Abbott Laboratories announced a five-dollar nasal swab test developed to ramp up testing in the US. This method detects antigens, specific proteins found on the surface of the virus. In the case of COVID-19, antigen tests often detect the infamous spike-protein, present during an active infection.
Because antigen tests are cheaper and faster—a result is usually delivered in about an hour— they can be a helpful tool for finding outbreaks and reducing the spread of COVID-19 in community settings such as schools and offices. The other advantage of this test is that it’s most likely to detect a positive case when people are at the peak of their infection (with lots of detectable virus or high “viral load”). That is typically when people are most infectious to others.
Although antigen tests are very specific, they are less sensitive, and thus less reliable, than PCR tests and may miss infections if “viral load” is too low (at the very beginning or near the end of an infection). When someone has symptoms of COVID-19, a positive antigen test is very likely to be accurate. However, if they test negative or are asymptomatic, it is possible a health care provider may recommend a follow up PCR test.
Some states count positive antigen results only as probable cases—and many don’t count them at all. This is a big downside, because without data reporting on these tests, their public health value is greatly reduced.
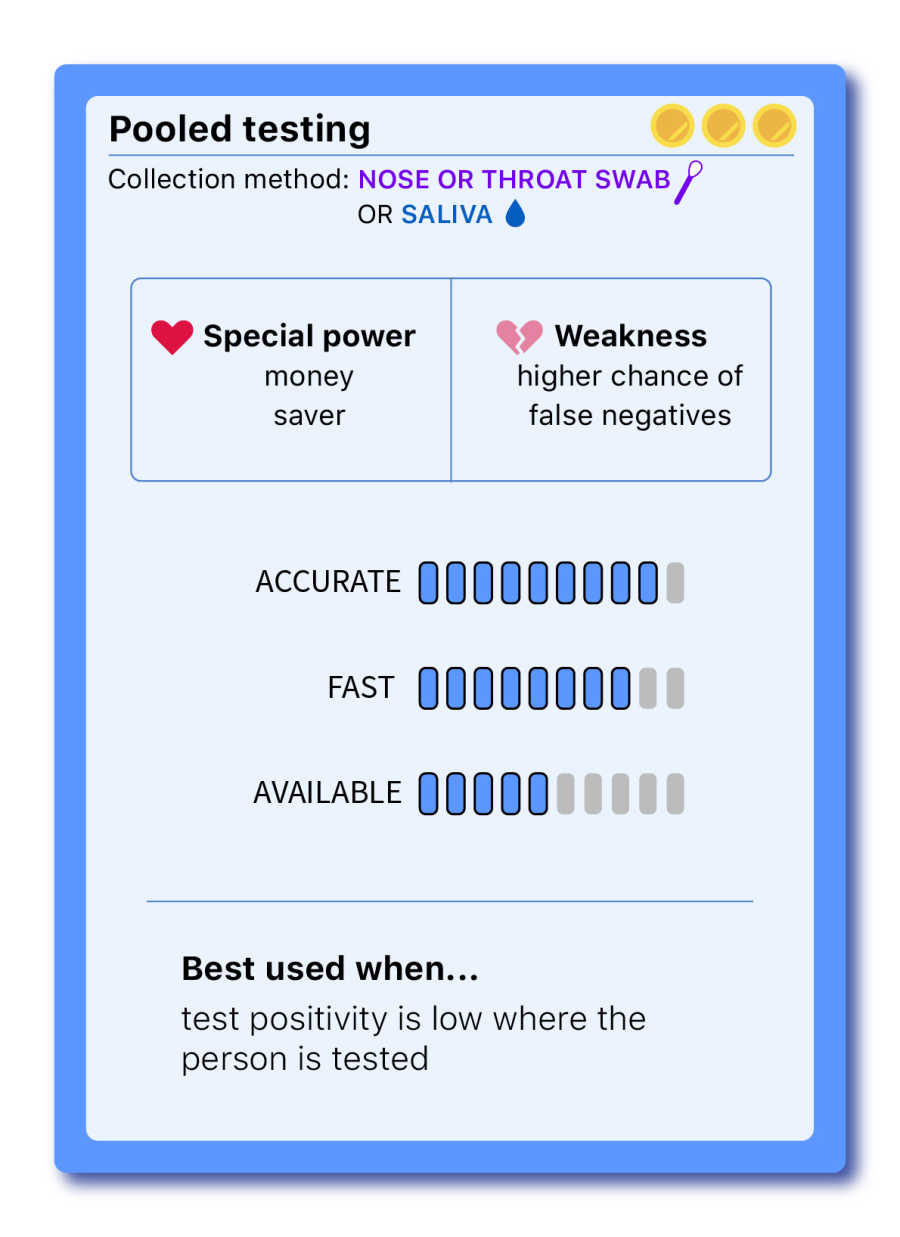
Pooled testing — (a strategy, not a test)
THE COST EFFECTIVE
In the pooled testing method, people are divided into groups and, instead of being tested individually, their samples are combined and tested all at once, using PCR tests. Each group gets a result. If a group tests negative, everyone in that group is presumed negative. If a group tests positive, all included samples are tested individually to identify the case or cases that are positive.
Approved by the FDA in mid-June, pooled testing significantly cuts down supplies and testing wait times, increasing testing utilization. While it reduces lab supply costs, it doesn't impact the price for patients or insurance billing. But testing multiple samples at once comes with a big catch: a higher chance of getting false negatives. Because a small portion of each sample is diluted with other samples, someone who carries a low viral load might not be detected in the pool. For that reason, experts disagree on how many samples can be tested at once without compromising the test sensitivity.
The other drawback of pooled testing is that it’s only advantageous when community transmission is low. If a state’s test positivity rate is too high, and most pools test positive, frequent retesting is necessary, outweighing the cost-effectiveness benefit.
Serology tests
Antibody
THE ONE THAT CAN SEE THE PAST
Unlike diagnostic tests, antibody tests—also known as serology tests—are not used to diagnose a current COVID-19 infection but rather to identify past infections by detecting the presence of antibodies (typically IgG and/or IgM) in a person’s blood sample.
Antibodies typically start to develop one to three weeks after infection, according to the CDC. That means that, if an infected patient is tested too early in their infection, it’s likely that they’ll get a negative test result. Since antibody tests are not intended to determine current infections or risk of infectiousness to others, these tests alone are not useful to prevent the spread of disease. It’s also worth noting that researchers still don’t know if the presence of these antibodies provides long-lasting immunity to the virus.
How the test types compare
The efficiency of test types varies according to the timeline of the infection. Diagnostic tests are better at detecting an active COVID-19 infection, whereas antibody tests detect recent or past infections.
Which test should you get?
The right test for each person depends on a series of factors, but it essentially comes down to the reason for getting tested. PCR is considered the most accurate method and is the recommended test by experts when a diagnosis is necessary. It’s especially useful when confirming or ruling out infection if reported symptoms are atypical for COVID-19. Consider this flow chart when determining when or why to get tested:
Sources: Centers for Disease Control and Prevention, Infectious Diseases Society of America, US Food and Drug Administration, Pooling of Upper Respiratory Specimens Using a SARS-CoV-2 Real-time RT-PCR Assay Authorized for Emergency Use in Low-Prevalence Populations for High-Throughput Testing, Open Forum Infectious Diseases, Volume 7, Issue 11, November 2020, ofaa466.
Ann Dinh, Elizabeth Eads, Erin Kissane, Lindsey Shultz, Michael Huynh, Rushmin Khazanchi, and Tong Wang contributed to this story.
Methodology: Tests scores of accuracy, turnaround times, and availability are calculated based on the lowest and the highest value across all tests. The accuracy score is an average of sensitivity and specificity values, which vary based on healthcare provider and lab. The accuracy score starts at 80%—the minimum sensitivity required by the FDA for an antigen test—and goes up to 100%. Pooled testing accuracy is based on pools of four samples. Pooled testing availability is contingent on test positivity and does not include states that are only conducting wastewater pooled tests. Antibody tests can also be tested in pools, but this piece only considers pooled molecular tests (PCR), which are by far the most common type in the United States. One point was deducted from PCR’s availability score to account for supply shortages.

Júlia Ledur is a visual journalist and illustrator who leads the visualization team at The COVID Tracking Project. She is a former graphics reporter at Reuters.

Jessica Malaty Rivera has an MS in Emerging Infectious Diseases and is the Science Communication Lead at The COVID Tracking Project.
More “Testing Data” posts
How Probable Cases Changed Through the COVID-19 Pandemic
When analyzing COVID-19 data, confirmed case counts are obvious to study. But don’t overlook probable cases—and the varying, evolving ways that states have defined them.
20,000 Hours of Data Entry: Why We Didn’t Automate Our Data Collection
Looking back on a year of collecting COVID-19 data, here’s a summary of the tools we automated to make our data entry smoother and why we ultimately relied on manual data collection.

A Wrap-Up: The Five Major Metrics of COVID-19 Data
As The COVID Tracking Project comes to a close, here’s a summary of how states reported data on the five major COVID-19 metrics we tracked—tests, cases, deaths, hospitalizations, and recoveries—and how reporting complexities shaped the data.